Research
Introduction
Hematopoietic stem cells (HSCs) are rare self-renewing cells that can generate the whole blood system throughout life. They are widely used in clinics to treat a number of hematopoietic disorders. But the rareness of HSCs has been hindering the safer and broader use of HSCs in clinics. Each year, thousands of lives can not be saved through HSC transplantation due to the insufficient supply of HSCs. Despite decades of efforts, no culture systems are available to maintain and expand HSCs in vitro. Yet, in vivo, HSCs robustly self-renew. We believe that understanding the microenvironmental niche in vivo is the key to solve this unmet medical challenge. In the lab, we are focusing elucidating the niche mechanisms that maintain HSCs in homeostasis and expand HSCs during development using genetically modified mouse models. As the bone marrow niche is increasingly appreciated as an important contributor to hematopoietic diseases, we also study the niche mechanisms in hematopoietic malignancies.
The Bone Marrow HSC Niche
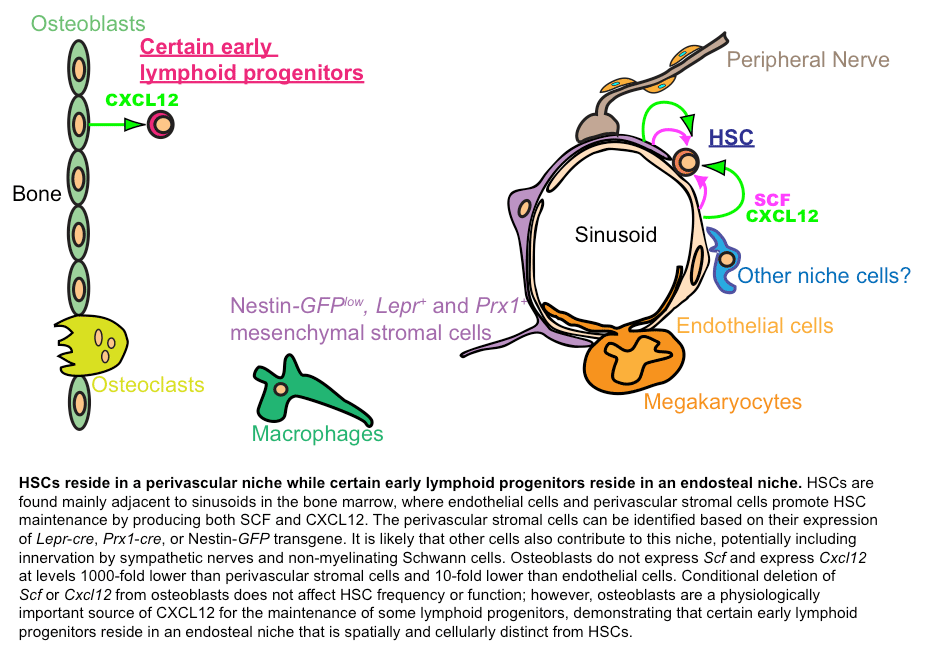
During homeostasis, HSCs reside in the bone marrow and are critically regulated by the niche. Although having long been recognized as an essential contributor to the regulation of HSCs, the components of the niche had been elusive. This has limited our ability to study the bone marrow niche mechanisms that regulate HSCs. Using functional genetics, our recent studies have discovered that HSCs reside in a perivascular niche, where endothelial cells and leptin receptor-expressing perivascular stromal cells are critical components by elaborating essential factors such as SCF and CXCL12 to maintain HSCs. Interestingly, we found that bone-lining osteoblast creates niche for lymphoid progenitors by generating CXCL12. The identification of the cellular components of the bone marrow niche has provided the opportunity to uncover novel niche mechanisms that maintain HSCs. Ongoing projects in the lab are aimed at identifying factors that regulate the bone marrow niche. A deep understanding of the bone marrow niche will help us better and safer use HSCs for bone marrow transplantation.
The Fetal Liver HSC Niche
The adult bone marrow niche maintains a steady pool of HSCs by keeping them in quiescent stage with occasional self-renewal divisions. But the bone marrow is not the only place where HSCs reside. During development, the fetal liver is a major hematopoietic organ where HSCs self-renew and expand dramatically. Comparing with the bone marrow niche, scarce information is available regarding the molecular and cellular mechanisms of the fetal liver niche. We are investigating the fetal liver HSC niche in vivo. Understanding how the fetal liver niche promotes extensively HSC self-renewal may hold the key to amply HSCs for clinical use.
The Niche in Hematopoietic Malignancy
Given the essential roles of the niche in HSC self-renewal regulation, it is not surprising that an abnormal niche contributes or even initiates hematopoietic malignancy. Mounting evidence suggests that many leukemia are propagated by leukemia stem cells (LSCs). These stem cell-like cells hijack the HSC maintenance mechanism and fuel the growth of the leukemia. They are responsible for relapse. Thus, eliminating LSCs holds the key in curing leukemia. The bone marrow niche is hypothesized to provide protection to LSCs. Recent evidence suggests that LSCs hijack and modify the bone marrow niche for their survival benefits. However, the exact niche mechanisms in leukemia and other hematopoietic malignancies are not clear. Ongoing projects in the lab are charactering the bone marrow niche in a number of hematopoietic malignancies. Understanding how LSCs interact with the bone marrow niche would greatly aid the identification of novel effective therapeutics to eradicate LSCs.